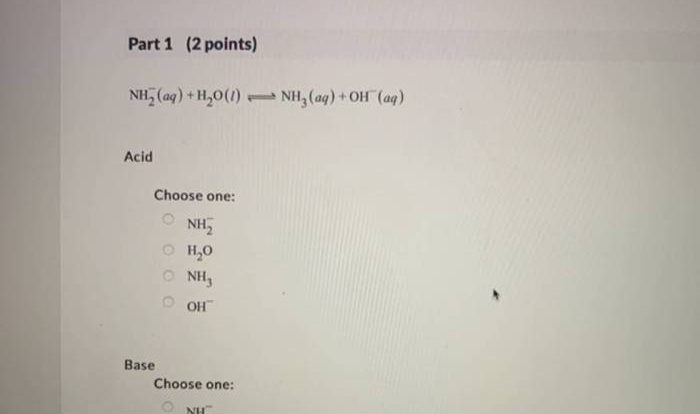
Embark on a journey of chemical exploration with our comprehensive single replacement reaction worksheet answers. Dive into the fascinating world of reactivity, oxidation, and reduction, and uncover the practical applications that shape our everyday lives.
Single replacement reactions, a cornerstone of chemistry, play a crucial role in understanding the behavior of elements and their interactions. This guide delves into the intricacies of these reactions, providing a thorough understanding of their mechanisms, applications, and significance.
Single Replacement Reactions: Definitions and Concepts
Single replacement reactions are chemical reactions in which one element replaces another element in a compound. They are significant because they provide a method for obtaining pure elements from their compounds and for understanding the reactivity of different elements.
The reactivity of an element refers to its tendency to undergo chemical reactions. In single replacement reactions, the more reactive element replaces the less reactive element in the compound. This concept is essential for predicting the feasibility and outcome of these reactions.
Types of Single Replacement Reactions, Single replacement reaction worksheet answers
- Metal-metal reactions: These reactions involve the replacement of one metal by another metal. The more reactive metal replaces the less reactive metal in the compound.
- Metal-nonmetal reactions: These reactions involve the replacement of a metal by a nonmetal. The more reactive metal replaces the less reactive nonmetal in the compound.
- Nonmetal-nonmetal reactions: These reactions are less common and involve the replacement of one nonmetal by another nonmetal. The more reactive nonmetal replaces the less reactive nonmetal in the compound.
Activity Series of Metals: Single Replacement Reaction Worksheet Answers

The activity series of metals is a list of metals arranged in order of decreasing reactivity. The most reactive metal is at the top of the series, while the least reactive metal is at the bottom. This series is important because it allows us to predict the feasibility of single replacement reactions.
If a metal is higher in the activity series than another metal, it will be able to replace that metal in a compound. For example, iron is more reactive than copper, so iron will be able to replace copper in a compound.
| Metal | Reactivity |
|---|---|
| Potassium | Most reactive |
| Sodium | |
| Calcium | |
| Magnesium | |
| Aluminum | |
| Zinc | |
| Iron | |
| Copper | |
| Silver | |
| Gold | Least reactive |
Reaction Mechanisms and Products
Single replacement reactions occur through a process of oxidation and reduction. Oxidation is the loss of electrons, while reduction is the gain of electrons.
In a single replacement reaction, the more reactive element is oxidized, and the less reactive element is reduced. For example, in the reaction between iron and copper sulfate, iron is oxidized to iron(II) ions, and copper is reduced to copper metal.
Fe + CuSO4→ FeSO 4+ Cu
The products of a single replacement reaction can be predicted by using the activity series of metals. The more reactive element will always replace the less reactive element in the compound.
Applications of Single Replacement Reactions
Single replacement reactions have a wide range of applications in various fields, including:
- Industrial processes:Single replacement reactions are used in the production of many metals, such as iron, copper, and aluminum.
- Laboratory techniques:Single replacement reactions are used in qualitative analysis to identify unknown metals.
- Everyday scenarios:Single replacement reactions are used in the rusting of iron and the tarnishing of silver.
Single replacement reactions are a versatile and important type of chemical reaction with numerous practical applications.
FAQ Overview
What are the key factors that determine the feasibility of a single replacement reaction?
The reactivity of the metals involved, as determined by their position in the activity series, plays a crucial role in predicting the feasibility of a single replacement reaction.
How can single replacement reactions be used to extract metals from their ores?
In metallurgy, single replacement reactions are employed to extract metals from their ores by displacing the less reactive metal with a more reactive one.
What are the safety considerations when working with single replacement reactions?
Single replacement reactions involving certain metals, such as sodium or potassium, can be highly exothermic and release flammable hydrogen gas, necessitating proper safety precautions and handling techniques.